Showing posts from February 18, 2011Show all
Metabolism of Fats and Proteins and Control of Metabolism and Metabolic Pool
Faizan Bhatti
5:36 PM
Catabolism of glucose is most common metabolic pathway in cells. Animals are consumed…
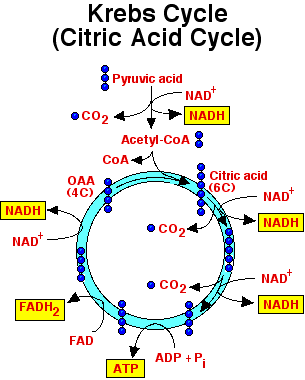
Kreb’s Cycle (OR) Citric Acid Cycle (OR) Tri-Carboxylic Acid Cycle (TCA)
Faizan Bhatti
5:21 PM
During metabolism the synthesis and breakdown of different organic compounds takes pl…
Popular Posts
Menu Footer Widget
Copyright ©
Notes for Pakistan




Social Plugin