Showing posts from November 11, 2010Show all
Energy, its Forms and Laws of Energy Transformation
Faizan Bhatti
3:39 PM
Energy is the capacity to work. Work is the transfer of energy. Energy can also be tra…
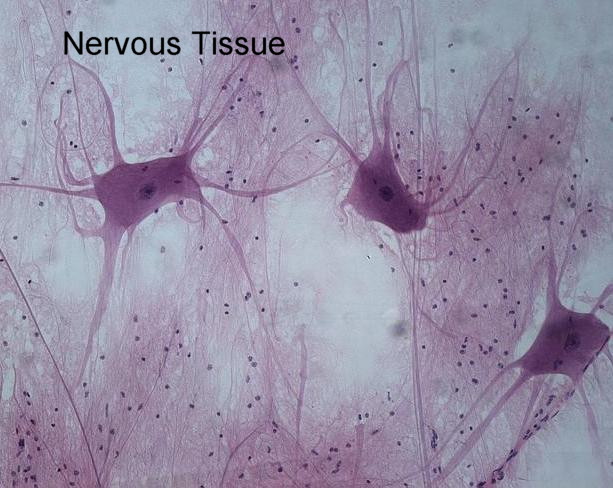
Nervous and Muscle tissue
Faizan Bhatti
3:36 PM
Nervous Tissue: It is composed of several different types of cells but two are i…
Popular Posts
Menu Footer Widget
Copyright ©
Notes for Pakistan




Social Plugin