RNA, its Structure and Kinds
RNA is Ribo nucleic acid. It occurs in the nucleolic, chromosomes and cytoplasm. About…
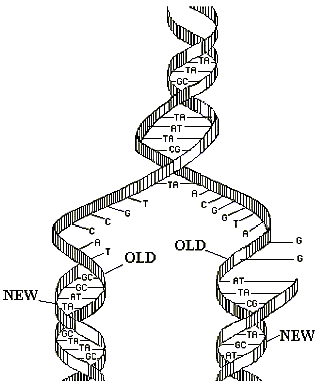
Replication of DNA
Essential property of genes is self duplication or replication. DNA possesses this pro…
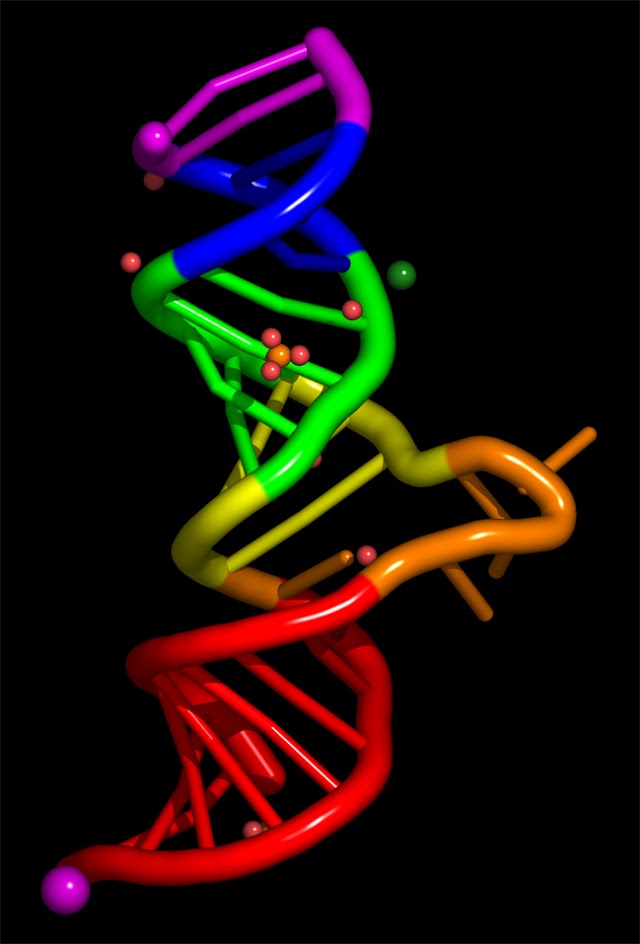
DNA, its Functions and Watson Crick Model of DNA
DNA is present in the chromosomes of the nucleus i.e. nuclear reticulum. DNA is double…
Nucleic Acids
22 years old, Swiss physician and chemist Friedrich Miescher isolated a substance f…
Describe Proteins, its structure, different function and types
--> Proteins are organic compounds of living organisms making 55 – 85% of …
Carbohydrates and their Classification
Carbohydrate literary means hydrated carbon. Carbohydrates are composed of carbon, h…
Acids, Bases and Buffers
Electrolyte is a substrate that conducts electricity when in solution, such as sodium …
Compounds and Molecules as Aggregates of Atoms
In addition to being an element, a substance can also be a compound. Compound is compo…
Atoms and Elements as Building Blocks
Matter occupies space and has mass. It includes solid, liquids and gases of the enviro…
Scientific Method
Science is an organized and systematic knowledge which is gathered through observat…
Environment and World Resources
Common environment unites life. In some parts of the world, the land is recovering fro…










Social Plugin